Preclinical development of an adoptive T cell therapy strategy to treat patients with non-small cell lung cancer
Principal Investigators:
Dr. phil. nat. Sean Hall, Inselspital, Bern University Hospital, Division of General Thoracic Surgery. For more information, please visit the website.
Co-Investigators:
Prof. Dr. phil. nat. Eliane J. Müller, Head Molecular Dermatology & Stem Cell Research, University of Bern & Insel, Bern University Hospital, Department of Dermatology, Insel and Department for BioMedical Research, Medical Faculty and Institute of Animal Pathology, Vetsuisse Faculty
Prof. Dr. med. Gabriela M. Baerlocher, Department of Hematology and head of Hematological Central Laboratory, Inselspital, Bern University Hospital, Hematology research group, Department for Biomedical Research, University of Bern.
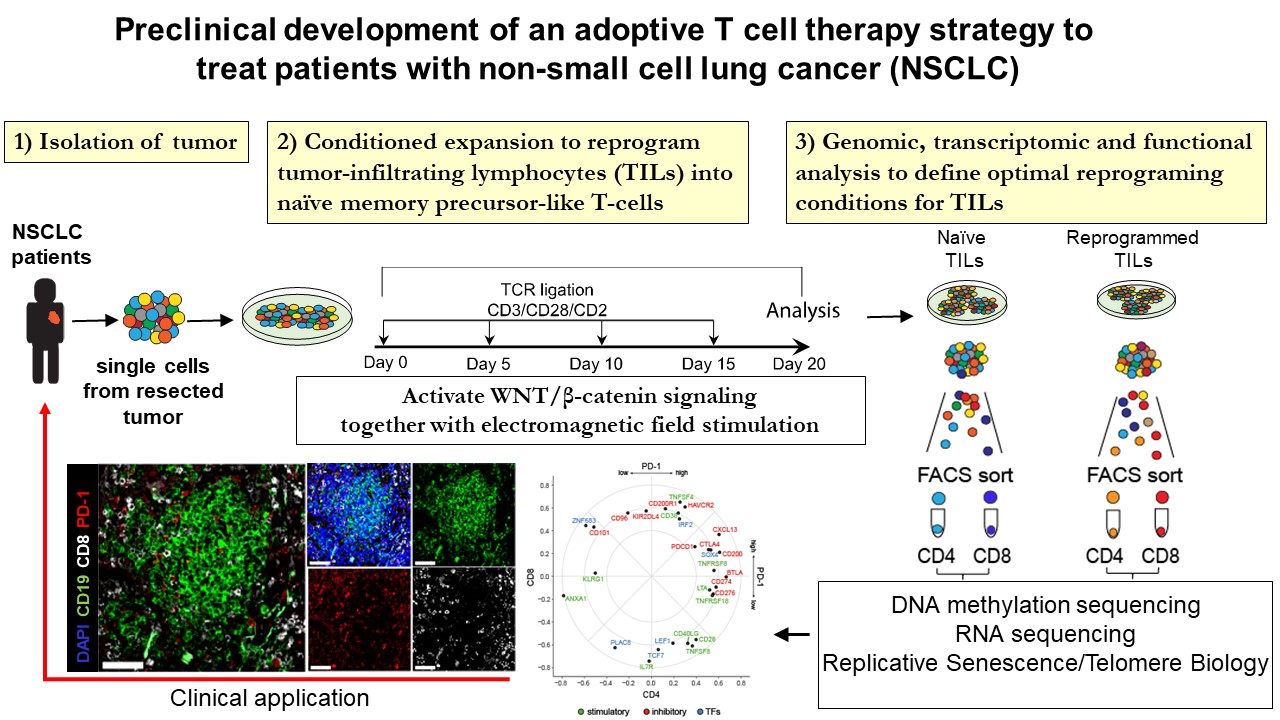
Adoptive T cell therapy (ACT), which involves ex vivo expansion and reinfusion of naturally occurring antigen-specific tumor infiltrating lymphocytes (TILs), represents a transformative living immunotherapy for the treatment of cancer. The majority of TIL-based ACT products are generated from terminally differentiated effector memory T cells resulting from repetitive rounds of TCR ligation during the ex vivo expansion phase. In lung cancer patients, we previously demonstrated that effector memory TILs show hallmarks of exhaustion coupled with downregulation of Wnt/β-catenin signaling and reduced expression of TCF7, an end effector of Wnt signaling. However, the presence of less differentiated T cells within the ACT product favours the generation of persistent and effective T cell memory populations and more favourable response rates, which has been linked with intact Wnt/β-catenin signaling pathway and longer telomeres. Therefore, the objective of this proposal is to test a novel intervention aimed at targeting Wnt/β-catenin to generate ex vivo expanded TILs with renewed and sustained functional ability to eliminate malignant lung cancer cells. The results of this project could set the basis for a clinical phase I/II study using a precision-based treatment strategy with optimized tumor-specific T cells against non-small cell lung cancer.